News
Fatal Protein Interactions May Explain Neurological Diseases
Published September 03, 2008
Media Contact:
Debra Kain,
ddkain@ucsd.edu or 619-543-6163
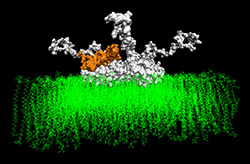
The team, led by Eliezer Masliah, M.D., professor of neurosciences and pathology in the UC San Diego School of Medicine, found that "fatal" or abnormal interactions among the a-synuclein protein (a-syn, involved in Parkinson's disease) and Abeta amyloid (Aβ, which leads to the plaques associated with Alzheimer's disease) interact and form unique "hybrid" complexes. These hybrid abnormal protein interactions result in combined neurodegenerative diseases.
"Clinically, we knew that having one neurological disease, such as Alzheimer's, put patients at risk for another neurological disease in combination with it, for example, Parkinson's disease or frontotemporal dementia. But as doctors and scientists, we didn't understand why this occurred until now," Masliah said. Through computer modeling, they discovered that when the Aβ and a-syn interacted they formed a new hybrid protein with a small hole called a "nanopore" that alters neuronal activity. Masliah described the model of the hybrid complex as being "like looking at a boat with holes in it. Because we can now see the holes, we can learn how to stop the leak."
Misfolding and aggregation of neuronal proteins has been proposed to play a critical role in the development of neurodegenerative disorders, including the leading disorders in the aging population - Alzheimer's disease and Parkinson's disease - which result in dementia and movement disorders. More than five million Americans live with such neurological conditions, and it is estimated that this country alone will see a 50 percent increase in Alzheimer's and Parkinson's disease alone by the year 2025.
In Alzheimer's, Aβ accumulates in the intracellular and extracellular spaces of the brain, leading to the formation of plaques. In Parkinson's, intracellular accumulation of an abundant synaptic protein, a-syn, results in the formation of characteristic foreign substances called "Lewy bodies." The mechanisms through which Aβ and a-syn interactions might lead to additional neurodegeneration have been the subject of intense scientific investigation, according to Masliah.
Working with researchers at the SDSC, Masliah and colleagues, including first author Igor Tsigelny from the Department of Chemistry and Biochemistry, developed a dynamic model using computer simulations. These included the so-called "molecular dynamics process," which allows insight into molecular motion on an atomic scale. Used to determine the properties of complex systems that contain a vast number of particles through use of numerical methods, molecular dynamics allowed the team to model how the abnormal neuronal proteins docked with other proteins or with cell membranes, and to measure the energies of interaction.
"This sort of modeling, to determine the structure of these complexes, was never before possible," said Masliah, adding that it was only possible at UC San Diego with its strong culture of scientific collaboration and the computing power of the San Diego Supercomputer Center. "With this novel technology, we have come to a new understanding of these combined neurological diseases, and have a model for developing new drugs to treat them."
These studies were supported by electron microscopy, along with cell and tissue studies of both mice and human brains, to characterize the nature of the interaction between the two proteins.
Co-investigators on this paper, all at UC San Diego, include first author Igor F. Tsigelny, Department of Chemistry and Biochemistry and the San Diego Supercomputer Center; Jason X.-J. Yuan and Oleksandr Platoshyn, Department of Medicine; Leslie Crews, Department of Pathology; Paula Desplats, Gideon M. Shaked, Hideya Mizuno, Brian Spencer, Edward Rockenstein and Margarita Trejo, Department of Neurosciences; and Yuriy Sharikov, San Diego Supercomputer Center.
The study was funded in part by the National Institutes of Health, IBM under its Institutes of Innovation program as well as computational support on its BlueGene computers at the San Diego Supercomputer Center and at the Argonne National Laboratory.