News
Unlocking Sustainable Battery Solutions Using San Diego Supercomputer Center Resources
Published May 14, 2024
Kimberly Mann Bruch, SDSC Communications
Researchers from the New Jersey Institute of Technology (NJIT) are making strides in the quest for sustainable battery technologies using U.S. National Science Foundation (NSF) ACCESS allocations on Expanse at the San Diego Supercomputer Center (SDSC) at UC San Diego.
NJIT Associate Professor of Mechanical and Industrial Engineering Dibakar Datta most recently published a study in the Royal Society of Chemistry Energy Advances journal that focused on addressing the pressing issue of the limited global supply of lithium, a key component in lithium-ion batteries (LIBs), which are widely used in everyday applications.
“As an alternative to lithium, scientists have turned their attention to earth-abundant and cost-effective multivalent metals such as aluminum and calcium, but a major challenge lies in finding suitable materials to host these multivalent ions in battery systems,” Datta explained. “Our research used ACCESS allocations on Expanse to closely examine a specific category of microparticles known as open-tunneled oxides, characterized by integrated one-dimensional channels or nanopores.”
Datta said that so far, the research has shown that niobium tungsten oxide (NTO) and molybdenum vanadium oxide (MoVO) have emerged as promising candidates. Specifically, his team’s study revealed that the MoVO structure boasts a larger surface area and different shapes—allowing it to accommodate a greater number of multivalent ions compared to NTO. Specifically, MoVO can adsorb ions such as calcium, lithium and aluminum with adsorption potentials ranging from around four to five electron volts (eV).
“However, the adsorption potential for hexagonal channels of aluminum ions drops to 1.73 eV due to limited channel area,” Datta said. “Meanwhile, the NTO structure exhibits insertion/adsorption potentials of 4.4 eV, 3.4 eV and 0.9 eV for lithium, calcium and aluminum ions, respectively.”
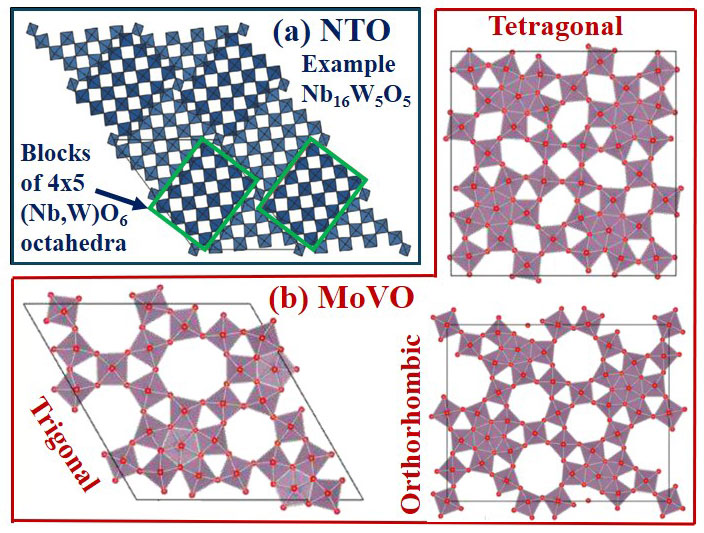
Porous materials such as these could potentially be a game-changer in the energy sector. This figure showcases open-tunnel oxides: (a) Niobium Tungsten Oxides (NTO) and (b) Various Molybdenum Vanadium Oxides (MoVO). Credit: New Jersey Institute of Technology
Generally, he said that it appeared that calcium ions are more readily adsorbed than aluminum ions in both MoVO and NTO structures. Hence, the team examined the role of charge transfer and ion size in the insertion of multivalent ions such as calcium and aluminum into MoVO and NTO systems – using Bader charge analysis and charge density plots.
They found that the vast compositional possibilities of open-tunnel oxide materials posed a challenge to their exploration for battery applications and that traditional experimental trials and quantum-based simulations were impractical for navigating this complexity.
“To address this challenge, we employed data mining and machine learning techniques using ACCESS allocations on Expanse to uncover innovative transition metal oxide materials,” Datta said. We compared two machine learning algorithms—one utilizing descriptors and the other employing graphs—to predict the synthesizability of new materials within a laboratory setting.”
He said that the final results provided valuable insights into the exploration of alternative naturally occurring multiscale particles, offering promising potential for the utilization of multivalent ions in battery-related contexts.
“This research represents a significant step forward in the quest for sustainable and efficient battery technologies crucial for meeting the growing demand for energy storage solutions in modern society,” said SDSC Director Frank Wuerthwein. “We are pleased that the NJIT team was able to use Expanse for their study and look forward to working with them on expanding this research even further.”
The study was funded by an NSF CAREER award (no. 2237990). Computational work on Expanse was funded by NSF ACCESS (grant no. DMR180013).

